Micronized sulphur powder for rubber
Micronized sulphur powder for rubber / powder sulphur rubber grade
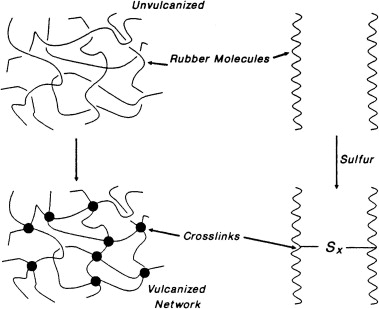
The role of sulphur in vulcanisation of rubber is, it reacts with these chains and forms disulphide (or similar) bonds. These new bonds join two of the hydrocarbon chains together. In this manner a mesh like network is formed which is stronger than the separate hydrocarbon chains in natural rubber. All of this comes about by the formation of the disuphide
Rubber makers sulfur which is also called vulcanizing agent or vulcanization agent is an important product that is used in vulcanization step of rubber and tyre manufacturing companies.Vulcanization is a chemical process that converts natural rubber and other polydiene elastomers into cross-linked polymers. The most common vulcanization agent is sulfur(powder sulphur, fine sulfur). It forms bridges between individual polymer molecules when heated with rubber. Often a catalyst and initiator is added to accelerate the vulcanization process. The cross-linked elastomers have much improved mechanical properties. In fact, unvulcanized rubber has poor mechanical properties and is not very durable.
Insoluble sulfur powder is amorphous form of sulfur made from the heat-polymerizing of powder sulfur, also can be obtained by reacting sulfureted hydrogen with sulfur dioxide. Insoluble sulfur is macromolecule polymer, and there are several thousand of sulfur atoms in its molecular chains. Since it doesn't dissolve in carbon disulfide, it is called insoluble sulfur or polymeric sulfur. Insoluble sulfur is an important rubber additive agent. It improves product quality, wear ability and resistance to both fatigue and ageing. In addition to being universally recognized as the best vulcanizing agent, it is widely used in the manufacture of tire, rubber pipe, shoes, cable and wire insulating materials, latex, automobile rubber parts and is also a necessary component of belt tires.
The polysulfide crosslinks formed by these reactions may contain four to six sulfur atoms at low temperatures whereas at higher reaction temperatures shorter sulfur bridges are formed.
Vulcanization of rubber by sulfur powder (powder sulphur) alone is extremely slow and can take several hours at elevated temperatures (140°C or more). This is problematic because long exposure to temperature and oxygen leads to oxidative degradation which, in turn, results in poor mechanical properties. It is also not very economical. To minimize rubber degradation and to speed-up the vulcanization process, accelerators are usually employed. An accelerator is defined as a compound that increases the speed of vulcanization and that enables vulcanization to proceed at lower temperature and with greater efficiency. Accelerator also decreases the amount of sulfur needed to cross-link the polyline thus improving the aging properties of the vulcanized rubber.